| Nanotechnology offers new strategies to enable minimally invasive and localized approaches for diagnosing and treating cancer, thereby avoiding the serious side effects and shortcomings of chemotherapy. For instance, it has been shown that often less than 1% of the administered drug molecules during chemotherapy enter tumor cells and bind to the nuclear DNA. Another complication is drug resistance of cancer cells. This actually is one of the main causes of failure in the treatment of cancer. | |
| Cancer researchers are looking to nanoparticles as a drug carrier capable of localizing and directly releasing drugs into the cell nucleus, thereby circumventing the multi drug-resistance and intracellular drug-resistance mechanisms to effectively deliver drugs to the vicinity of DNA, leading to a high therapeutic efficacy. | |
| Over the past decade, nanoparticles have been explored for a wide range of applications, including drug delivery vehicles, imaging contrast probes, and therapeutic agents. However, although increased therapeutic efficacy has been realized – and evidence that nanoparticles can affect intracellular function has been seen via fluorescent labels – there have been no reports on visualizing at nanoscale dimensions how nanoparticles interact with specific organelles. How nanoparticles interact with the cell nucleus, for example, will have implications not only for the fundamentals of cancer biology but also for the design of translational therapeutic agents. | |
| In a new breakthrough for nanomedicine cancer research, scientists have now reported the direct visualization of interactions between drug-loaded nanoparticles and the nucleus of a cancer cell. | |
| In a paper in the March 16, 2012 online edition of ACS Nano ("Direct Observation of Nanoparticle-Cancer Cell Nucleus Interactions"), Teri W. Odom, Board of Lady Managers of the Columbian Exposition Professor of Chemistry and Professor of Materials Science and Engineering at Northwestern University, and her team report two breakthrough results on the interaction of drug-loaded nanoconstructs with the cancer cell nuclei: | |
| 1) Nanoconstructs trafficked to the nucleus via a shuttling protein produced invaginations in the nuclear envelope at the site of the drug-loaded nanoparticles; and | |
| 2) Changes in nuclear phenotype – specifically increases in the number, depth, and shape of the nuclear envelope invaginations – were strongly correlated with changes in cell function. | |
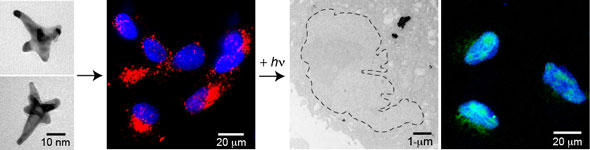 | |
| The image shows from left: (1) formation of gold nanostars and (2) delivery of AS1411 gold nanostars to locations near the cancer cell nuclei. (3, left) The release of AS1411 from gold nanostars via fs-pulsed excitation resulted in increased deformation of the cancer cell nucleus. (3, right) The released AS1411 also produced double stranded DNA breaks in the nucleus. (Reprinted with permission from American Chemical Society) | |
"We have reported the first imaging results on how drug-loaded nanoparticles affect nuclear phenotype," Odom tells Nanowerk. "The most surprising aspect of this work was that changes in nuclear phenotype were correlated with the biological response of the treated cancer cells." | |
| The team's two-component nanoconstructs consisted of gold nanostars and the single-stranded DNA aptamer AS1411. AS1411 has been tested as a chemotherapeutic agent because of its ability to strongly bind to nucleolin, a protein that is over expressed in cancerous cells. By blocking several functions of nucleolin, AS1411 can ultimately result in tumor cell death. | |
| Odom points out that, surprisingly, high resolution TEM images showed major changes in nuclear phenotype near the site of the nanoconstruct; the nuclear envelope was extremely deformed in over 60% of the tumour (HeLa) cells with nanoconstructs. | |
| To test whether the concentrated release of aptamer from the constructs could increase nuclear envelope folding further, the researchers used ultra-fast (femtosecond), light pulses to detached AS1411 from the surface of the gold nanostars. They found that this increased the number of local deformations in the nucleus. | |
| "That cancer cell function can be correlated with deformations in the nucleus suggests that major challenges in particle-based, nuclear-targeted therapy can be overcome," says Odom. "For example, complete internalization of nanoconstructs inside the nucleus is not necessary if induced physical changes in nuclear phenotype can disrupt nuclear functions. Also, because morphological effects are induced by nanoparticles outside the nucleus, there may be no limitation in the size or shape of nanoparticles that can achieve a similar response. These factors should provide insight into the development of new strategies to design drug-loaded nanoparticles with increased therapeutic efficacy." | |
| Currently, the researchers are testing this nanoconstruct in a variety of carcinomas in vitro. Because the shuttling protein nucleolin is over expressed on all proliferating cancer cells, they expect to observe high anti-cancer potency in a wide range of cancers. | |
| By Michael Berger. Copyright © Nanowerk Fonte: NanoWerk Spotlight |
